Catalase enzyme: basic features
Catalase - an enzyme found in almost allliving organisms. Its main function is to catalyze the decomposition reaction of hydrogen peroxide to substances harmless to the body. Catalase is of great importance for the vital activity of cells, as it protects them from destruction by active forms of oxygen.

General information
The enzyme catalase refers to oxidoreductases, a vast class of enzymes that catalyze the transfer of electrons from a reducing molecule (donor) to an oxidizing molecule (acceptor).
The optimum pH for catalase in humanthe body is about 7, however, the reaction rate does not change significantly at values of the hydrogen index from 6.8 to 7.5. The optimal pH for other catalases ranges from 4 to 11, depending on the type of organism. The optimum temperature also varies, for a person it is about 37about FROM.
Catalase is one of the fastest enzymes. Just one molecule of it can convert millions of hydrogen peroxide molecules into water and oxygen in a second. From the point of view of enzymology, this means that the catalase enzyme is characterized by a large number of revolutions.

Structure of the enzyme
Catalase is a tetramer of fourpolypeptide chains, each of which has a length of more than 500 amino acids. The enzyme has four groups of porphyry heme, due to which and reacts with the active forms of oxygen. Oxidized heme is a prosthetic group of catalase.
History of discovery
Catalase was not known to scientists until 1818,while Louis Jacques Tenar, a chemist who discovered hydrogen peroxide in living cells, did not assume that its destruction was due to the action of a previously unknown biological substance.

In 1900 the German chemist Oscar Lev first introducedterm "catalase" for the designation of a mysterious substance that decomposes peroxide. He also managed to answer the question, where the enzyme catalase is contained. As a result of numerous experiments, Oscar Lev revealed that this enzyme is characteristic of almost all animals and plant organisms. In a living cell, like many other enzymes, catalase is contained in peroxisomes.
In 1937, for the first time it was possible to crystallizecatalase from beef liver. In 1938, the molecular mass of the enzyme was determined to be 250 kDa. In 1981, scientists received an image of a three-dimensional structure of bovine catalase.
Catalysis of hydrogen peroxide
Despite the fact that hydrogen peroxide is a product of many normal metabolic processes, it is not harmless to the body.

The reaction of decomposition of hydrogen peroxide in living tissues:
2 H2O2 → 2 H2O + O2
Molecular mechanism of peroxide cleavagehydrogen catalase enzyme has not yet been studied. It is assumed that the reaction takes place in two stages - at the first stage, iron in the prosthetic group of catalase binds to the oxygen atom of peroxide, while one molecule of water is released. At the second stage, the oxidized heme reacts with another molecule of hydrogen peroxide, resulting in the formation of another molecule of water and one molecule of oxygen.
Due to this action of the enzyme catalase onhydrogen peroxide, the presence of this active substance in tissue samples is easy to determine. To do this, just add a small amount of hydrogen peroxide to the sample and observe the reaction. The presence of an enzyme is indicated by the formation of oxygen bubbles. This reaction is good because it does not require any special equipment or tools - it can be observed with the naked eye.
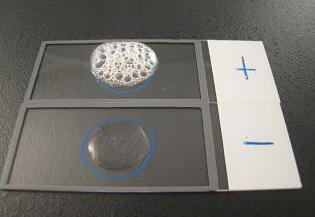
It is worth noting that the ion of any heavy metalcan act as a non-competitive inhibitor of catalase. In addition, all known cyanide behaves as a competitive catalase inhibitor, if there is a lot of hydrogen peroxide in the tissues. Arsenates play the role of activators.
Application
The degrading effect of the catalase enzyme on hydrogen peroxide has found application in the food industry - this enzyme removes from the milk H2ABOUT2 before cooking the cheese. Another application is special food packaging, which protects the products from oxidation. Catalase is also used in the textile industry to remove hydrogen peroxide from tissues.
It is used in small amounts inhygiene of contact lenses. Some disinfectants have hydrogen peroxide in the composition, and catalase is used to split this component before reusing the lenses.
Activity
The activity of the catalase enzyme depends on the ageorganism. In young tissues, the activity of the enzyme is much higher than in the old ones. With age, both in humans and in animals, the activity of catalase gradually decreases as a result of aging of organs and tissues.
According to a recent study, a decreaseactivity of catalase is one of the possible causes of graying of hair. Hydrogen peroxide is constantly formed in the human body, but does not harm - catalase quickly decomposes it. But if the level of this enzyme is reduced, it is obvious that not all hydrogen peroxide is catalyzed by the enzyme. Thus, it discolours the hair from the inside, dissolving natural dyes. This unexpected discovery is now being tested by researchers, and, perhaps, will play a role in the development of drugs that suspend hair graying.
</ p>