What is the oxygen valence in the compounds?
In order to determine the possible valuesthe valence of oxygen, we should study the position of the element in the periodic table, the main features of the structure of its atom. Such an approach is convenient in studying the question of which oxygen valence is typical, and which is uncharacteristic for it. In the most common compounds, the usual valence is II. This feature makes it possible to determine the number of bonds of another atom in the finished binary formulas involving oxygen.
What is the oxygen valence?
At the initial stage of the accumulation of knowledge aboutproperties and structure of substances, chemists thought that valence is the ability to bind a certain number of atoms to a molecule of matter. Many scientists, after the discovery of the element, tried to understand what is the valence of oxygen. The answer was obtained experimentally: oxygen adds two atoms of monovalent hydrogen to the chemical reaction, which means that it is bivalent. Representations of the chemical bond changed as knowledge about the structure of matter accumulated. In their theory of valence, G. Lewis and V. Kossel disclose the essence of chemical interaction from the point of view of the electronic structure. The researchers explained the ability of an atom to form a certain number of bonds by striving for the most stable energy state. If it is achieved, the smallest particle of the substance becomes more stable. In the theory and structures of Lewis much attention is paid to the role of external electrons participating in the creation of a chemical bond.
Features of oxygen placement in the periodic table
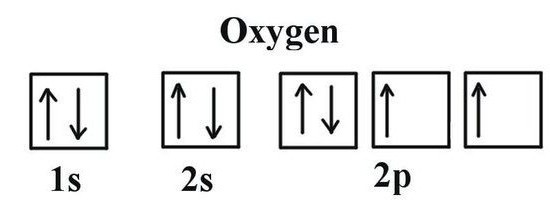
In order to determine which valence yoxygen, it is necessary to consider some features of its electronic structure. Oxygen leads the 16th group of the periodic table. The trivial name of the family of elements is "chalcogenes", they are referred to the VI (A) group by outdated classification. In the periodic table oxygen is in the cell under No. 8. The core contains 8 positive and as many neutral elementary particles. In the space of an atom, there are two energy levels that arise when 8 electrons move, of which 6 are external.

What is the relationship between the composition of the atom and the valence?
The last level of the oxygen atom contains 2unpaired electrons. Element is inferior to fluorine in terms of electronegativity (the ability to attract binding electron pairs to itself). In the formation of compounds with other elements, oxygen attracts to itself the total electronic density that has arisen in the molecule (except for fluorine electrons). Achieving the stable state of the outer shell is possible with the addition of two negative charges. This means that oxygen requires 2 electrons. The possible options are as follows: take one electron (valence II), select 2 electrons from another atom (valence II), do not take electrons from other atoms (valence 0). The typical behavior of oxygen is characterized by the second case. This way you can use to find out which oxygen is most typical in its common compounds. These include most of the oxides of metals and nonmetals.

How is valence manifested in compounds?
Oxygen is able to directlyinteract with many chemical elements. Known are its compounds with virtually all representatives of the periodic table (with the exception of inert gases: argon, helium, neon). In reaction with halogens, noble metals, oxygen can not directly enter, but Au oxides2O3, F2O, Cl2O7 and others exist (receive indirectly). For binary compounds in the formation of which oxygen participates, covalent bonding and polarity are characteristic. Valence in such molecules depends on the number of emerging pairs of electrons to which the nuclei of different atoms are attracted. In the vast majority of compounds, oxygen atoms participate in the creation of two covalent bonds. For example, in CO oxides2, R2ABOUT5, SO2, SO3, K2O, B2ABOUT3, Mo2ABOUT5 and in other molecules. In the hydronium cation H3O + oxygen exhibits an atypical valence III. The presence of the peroxo group -O-O- is due to the unusual nature of hydrogen peroxide H2ABOUT2. In this compound, oxygen exhibits its inherent valence II.

How to determine the valence of the elements?
The idea of the valence possibilities of oxygengives the Lewis structure - the chemical sign of the element, around which the electrons mark the outer layer. They are involved in the creation of molecules, are part of the common electronic pairs. The Lewis formula clearly demonstrates the oxygen valence, corresponding to the number of its unpaired electrons (2). The same result is provided by the use of graphic electronic structures. In two cells of the external energy level of oxygen, unpaired electrons are located (indicated by arrows in the formula). Information on the valence of oxygen makes it possible to determine the value for neighboring atoms from the finished binary compound formula. For this purpose, simple calculations are carried out. First, the number of O atoms is multiplied by the exponent of the usual valence oxygen. The resulting value should be divided by the index, which is indicated in the formula next to the chemical symbol of another element in conjunction with oxygen. Using a simple method, we calculate the valence of carbon and phosphorus in their oxides.
- We multiply the subscript to the right below the sign of 0 in CO dioxide2 to the typical valency of the element: 2 • 2 = 4. The resulting number is divided by the index indicated for carbon: 4/1 = 4. In dioxide CO2 carbon is in its highest valence state IV.
- The index at the bottom right of the chemical symbol of oxygen in phosphorus oxide P2ABOUT5 multiply by the typical valency of the O atom: 5 • 2 = 10. We divide this number by the index on the right in the formula below from the phosphorus atom: 10/2 = 5. In oxide, phosphorus is in its higher valence state V.




